
A breakthrough in RF technology, BioFuse™ tissue welding, is engineered to replace sutures and staples while delivering faster, stronger, and more reliable surgical closure.
The Tissue Welding Company
About the technology
What Is BioFuse?
BioFuse™ is a next-generation RF tissue sealing and welding technology engineered to transform how surgeons achieve sealing and closure. By eliminating the need for traditional sutures and metal staples, BioFuse™ delivers a fast, precise, and leak-proof connection between tissues—supporting better outcomes and faster healing.
01
No staples. No Sutures. No compromise.
BioFuse™ creates a continuous, gap-free tissue seal—reducing the trauma, leak risk, and scarring associated with traditional closure methods.
02
Smart Energy, Surgical Precision
Our proprietary RF algorithm continually adjusts in real-time (versus sampling), delivering consistent, controlled energy that adapts to tissue conditions during every procedure.
03
Clinically Proven. FDA Cleared.
Validated in 1,000's of procedures in Europe, our original vessel sealer is 510(k) cleared and we're preparing to submit an updated 510(k) application for our present generator and hand piece.
Surgical Stapler Market in the USA (2)
Surgical Staplers Sold in the USA in 2025 (3)
FDA received MDR's (product complaints/issues) for internal surgical staplers & staples (4)
Clinical & market Impact
A $6B Market, Ready for Disruption
Surgeons are seeking the next-generation solution that eliminates scarring, lowers infection rates, and supports evolving minimally invasive techniques.
1. Internal BioFuse data on procedures in Europe
3. BioFuse internal data from an iData report
Why it Matters
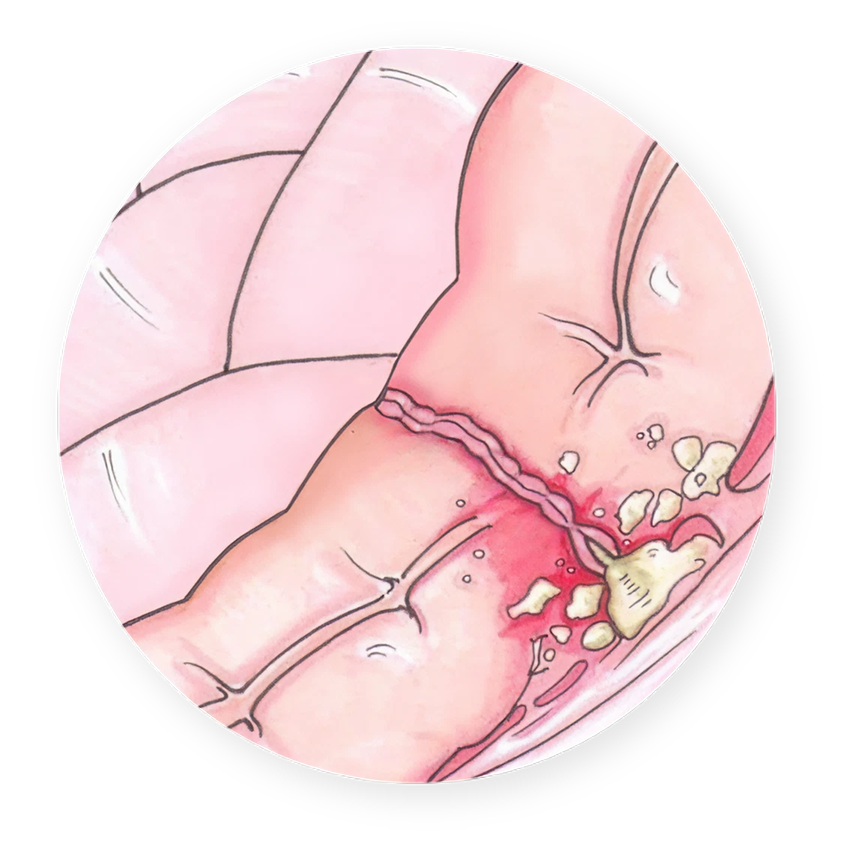
Limitations of Surgical Staples
- Staples high leak risks 3% – 12%*
- Staples in the body:
•intrinsically leave gaps
•are initially rejected as foreign body
•cause a period of weakness
•can lead to infection and sepsis
•can result in repeat procedures
*Source: IGIE Journal, 2022
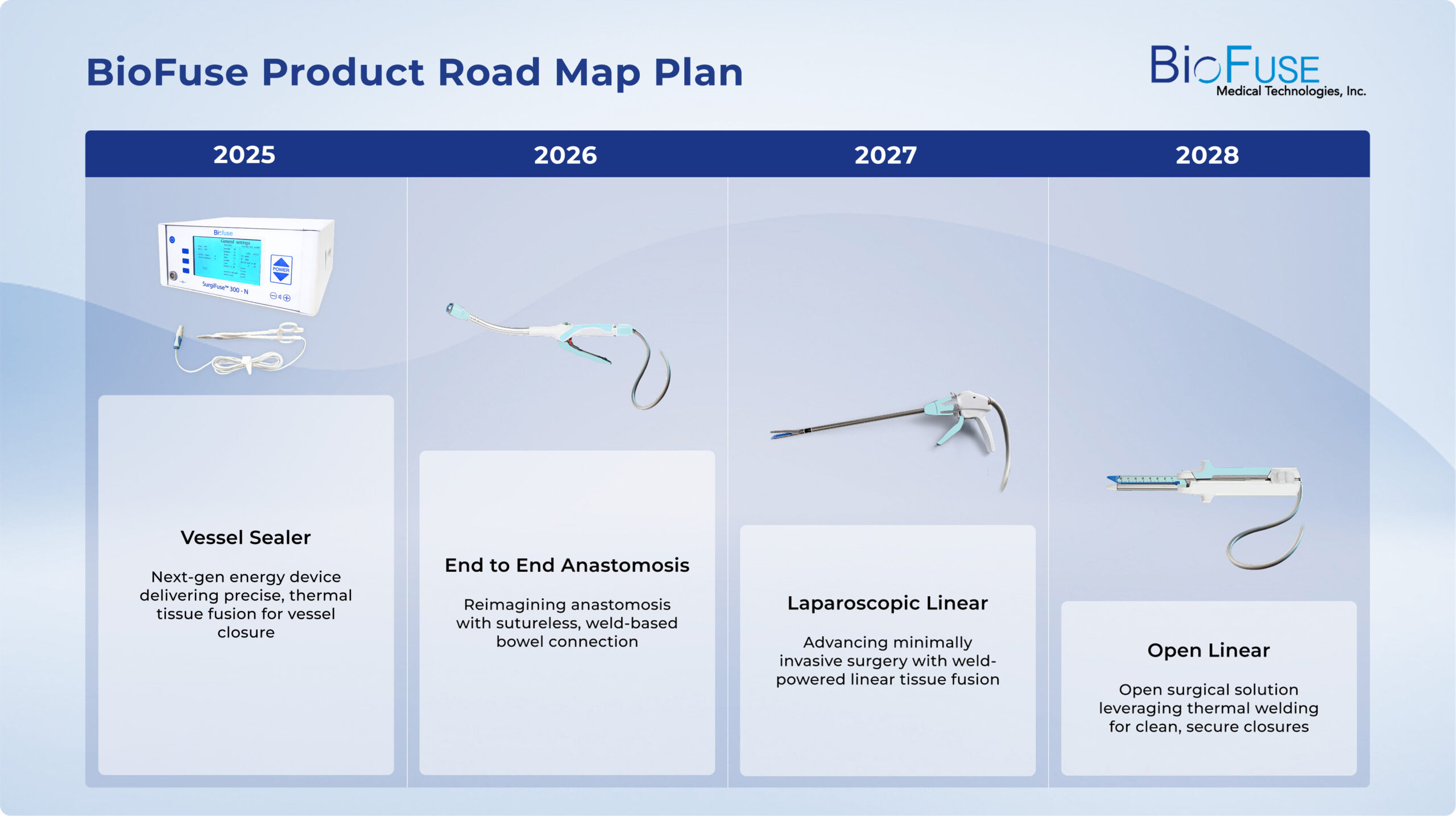
Pipeline & IP Strength
More than a product - a platform
BioFuse™ has a robust pipeline of future products, backed by strong IP protection with proprietary trade secrets. This isn’t just a device — it’s the foundation for a new era in surgical energy delivery for tissue welding.
let's transform surgery together
Whether you’re a provider, investor or partner — we want to hear from you.

Disclaimer: The product and procedures depicted above have not been cleared or approved by the U.S. Food and Drug Administration (FDA) and are presented solely for informational purposes. These materials are intended for healthcare professionals and do not constitute promotion or an offer for sale within the United States.
Contacts
- BioFuse Medical Technologies, Inc. PO Box 154, Melbourne, FL 32902
- info@biofusemedical.com
- 877.466.2434
© BioFuse™ Medical Technologies, Inc. All Right Reserved 2025.















